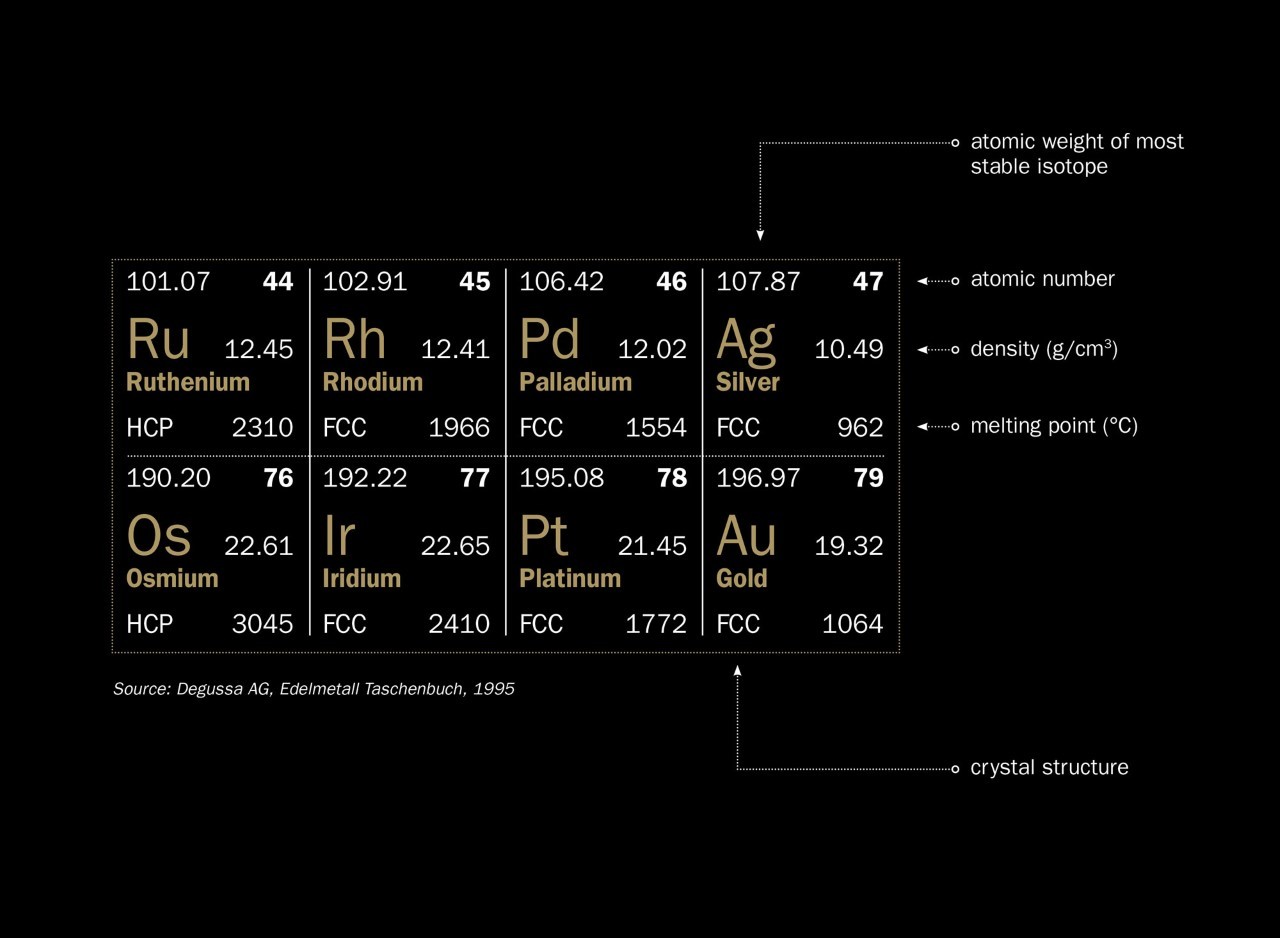
Gold has a density of 19.32 grams per cubic centimeter. This makes it one of the densest elements on Earth.
Gold’s high density contributes to its significant value and desirability. Found in various forms, from nuggets to microscopic particles, gold’s density ensures its weight is always noticeable. This characteristic also makes it ideal for various industrial applications, including electronics and aerospace.
Gold’s density plays a crucial role in its use in jewelry, providing a substantial feel that signifies luxury. The high density of gold also aids in its identification and verification, distinguishing it from less dense metals. Understanding gold’s density helps in appreciating its unique properties and widespread uses.

Credit: www.daviddarling.info
Gold’s Density
Gold has always fascinated humans due to its unique properties. One of the most intriguing aspects is its density. This precious metal is not only valuable but also incredibly dense. Let’s explore what makes gold so dense and how it compares to other metals.
Atomic Structure
The atomic structure of gold plays a significant role in its density. Gold atoms are tightly packed together. Each gold atom has 79 protons and 118 neutrons. These particles give gold its heavy weight. The atomic number of gold is 79, making it a heavy element. Gold atoms form a dense, crystal lattice structure.
Comparison With Other Metals
Gold’s density stands out among other metals. Let’s compare gold with some common metals:
| Metal | Density (g/cm³) |
|---|---|
| Gold | 19.32 |
| Silver | 10.49 |
| Iron | 7.87 |
| Aluminum | 2.70 |
Gold is almost twice as dense as silver. It is more than twice as dense as iron. It is over seven times denser than aluminum. This high density makes gold feel very heavy.
Gold’s density contributes to its value. It makes gold easy to shape and form. This property has made gold desirable for jewelry and coins throughout history.
Measuring Gold’s Density
Gold is a unique metal with a very high density. Understanding its density is crucial for various applications. Let’s explore how to measure gold’s density effectively.
Tools And Techniques
Measuring the density of gold requires precise tools and methods. Here are some common tools used:
- Hydrostatic Balance: This tool measures gold’s weight in air and water.
- Archimedes’ Principle: This technique involves submerging gold in water.
- Electronic Densitometer: A modern device providing accurate density readings.
These tools help in determining gold’s density accurately. Each method has its own advantages.
Practical Applications
Understanding gold’s density is essential in many fields. Here are some practical applications:
- Jewelry Making: Jewelers need to know gold’s density for crafting pieces.
- Investment: Investors verify gold’s purity using density measurements.
- Industrial Use: Industries use gold in electronics and need precise density data.
Knowing gold’s density ensures quality and authenticity in these applications.
Gold’s Unique Properties
Gold is a unique metal with amazing properties. It is dense, shiny, and does not rust. These properties make gold very special. Let’s explore some of these unique properties.
Conductivity
Gold is an excellent conductor of electricity. It allows electricity to flow easily. Many electronic devices use gold. This is because gold is reliable and does not corrode.
Here is a table showing gold’s conductivity compared to other metals:
| Metal | Conductivity |
|---|---|
| Gold | Excellent |
| Silver | Excellent |
| Copper | Very Good |
| Aluminum | Good |
Malleability
Gold is very malleable. This means it can be hammered into thin sheets. A single gram of gold can be beaten into a sheet one square meter large.
Here are some interesting facts about gold’s malleability:
- Gold can be stretched into a thin wire.
- Gold sheets can be used for decoration.
- Gold leaves are used in art and architecture.
Gold’s malleability makes it ideal for jewelry. It can be shaped into many designs.
Credit: www.lbma.org.uk
Uses Of Dense Gold
Gold is known for its density and unique properties. This precious metal plays a crucial role in various fields. Let’s explore the uses of dense gold in different sectors.
Jewelry
Dense gold is perfect for making jewelry. Its weight and shine make it valuable. Gold’s density helps it resist wear and damage. This ensures that gold jewelry lasts a long time. Gold’s malleability allows intricate designs. You can find gold in rings, necklaces, and bracelets. Gold is also used in cultural and religious artifacts.
Electronics
Dense gold is essential in the electronics industry. Its excellent conductivity is crucial for electronic components. Gold is used in connectors, switches, and relay contacts. It ensures reliable and fast connections. Gold’s resistance to corrosion increases the lifespan of devices. Mobile phones, computers, and other gadgets all use gold. The density of gold also helps in shielding sensitive electronic parts.
The Science Behind Gold’s Density
Gold has fascinated humans for centuries. Its density is a key feature. Density is the mass of an object divided by its volume. This property makes gold unique among metals. Let’s dive into the science behind gold’s density.
Historical Discoveries
Ancient civilizations knew gold was heavy. They used it for coins and jewelry. But they didn’t know why it was so dense. In Archimedes’ time, people began to understand density. Archimedes discovered the principle of buoyancy. This principle helped measure density. Gold became even more valuable because of its known properties.
Modern Research
Today’s scientists use advanced tools to study gold’s density. X-ray crystallography helps us understand gold’s atomic structure. Gold atoms are tightly packed. This tight packing increases its density. The atomic number of gold is 79. This high number contributes to its weight. Gold’s density is 19.32 grams per cubic centimeter. This value is higher than many other metals.
Below is a table comparing the density of gold with other metals:
| Metal | Density (g/cm³) |
|---|---|
| Gold | 19.32 |
| Silver | 10.49 |
| Copper | 8.96 |
| Iron | 7.87 |
Modern research shows gold’s atomic structure is key to its density. This research helps in many fields. From jewelry making to electronic devices, gold’s density is crucial.

Credit: www.smart-elements.com
Frequently Asked Questions
What Is The Density Of Gold?
Gold has a density of 19. 32 grams per cubic centimeter.
How Is Gold’s Density Measured?
Gold’s density is measured by dividing its mass by its volume.
Why Is Gold So Dense?
Gold’s high atomic number and tightly packed atomic structure contribute to its density.
Is Gold Denser Than Lead?
Yes, gold is denser than lead, which has a density of 11. 34 grams per cubic centimeter.
How Does Gold’s Density Affect Its Value?
Gold’s density contributes to its weight, making it valuable in smaller quantities.
Can Gold’s Density Vary?
Pure gold’s density remains constant, but alloys may have different densities.
Conclusion
Gold’s density makes it unique among metals. This trait contributes to its value and various applications. Understanding gold’s density deepens appreciation for this precious metal. Whether in jewelry or electronics, gold remains unparalleled. Explore the fascinating world of gold and its remarkable properties.
Stay curious and continue learning about this extraordinary element.

Rakib Sarwar is a seasoned professional blogger, writer, and digital marketer with over 12 years of experience in freelance writing and niche website development on Upwork. In addition to his expertise in content creation and online marketing, Rakib is a registered pharmacist. Currently, he works in the IT Division of Sonali Bank PLC, where he combines his diverse skill set to excel in his career.
