Uranium has a density of about 19.1 grams per cubic centimeter. This makes it one of the densest naturally occurring elements.
Uranium is a heavy metal with significant industrial and scientific importance. Its high density is a notable characteristic, making it heavier than lead. Uranium’s primary use is in nuclear reactors and weapons, where it serves as a critical fuel source.
This element is also used in various applications, including counterweights, radiation shielding, and even in some medical devices. Its dense nature allows for compact energy storage, which is vital in nuclear technology. Understanding uranium’s density helps in appreciating its role in both energy production and national defense.
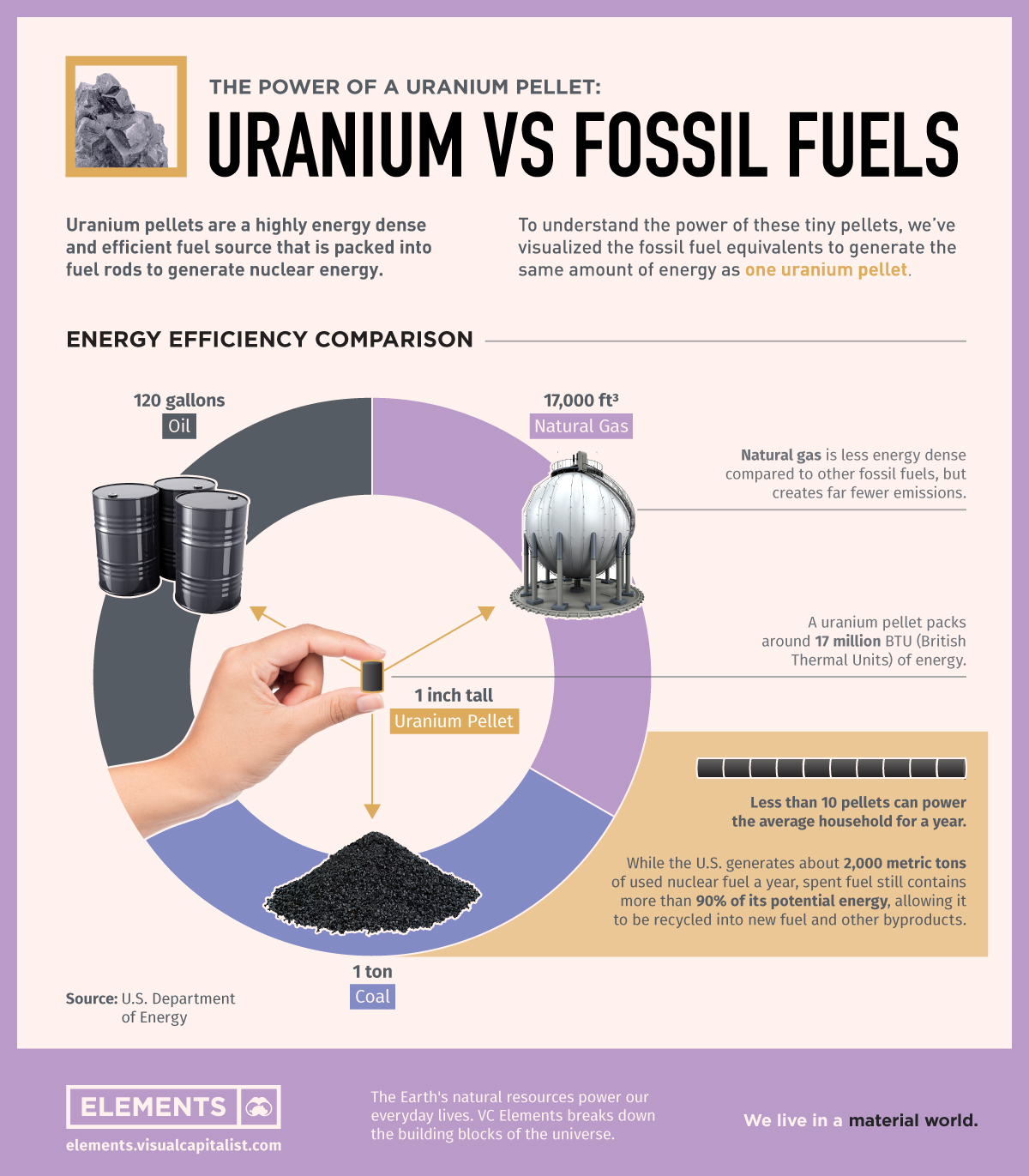
Credit: elements.visualcapitalist.com
Uranium’s Atomic Structure
Uranium is one of the densest elements found in nature. This makes it incredibly unique. The secret lies in its atomic structure. Let’s explore this in detail.
Atomic Composition
Uranium has an atomic number of 92. This means it has 92 protons in its nucleus. Protons are positively charged particles. Uranium also has neutrons. Neutrons have no charge. They are neutral particles.
The number of neutrons can vary. This variation leads to different isotopes. The most common isotope is Uranium-238. It has 146 neutrons. Another isotope is Uranium-235. It has 143 neutrons.
Uranium atoms also have electrons. These are negatively charged particles. They orbit the nucleus. Uranium has 92 electrons. They are arranged in different energy levels or shells.
| Particle | Charge | Number in Uranium |
|---|---|---|
| Protons | +1 | 92 |
| Neutrons | 0 | 143-146 |
| Electrons | -1 | 92 |
Isotopes Of Uranium
Isotopes are different forms of the same element. They have the same number of protons. They have different numbers of neutrons. Uranium has several isotopes.
- Uranium-238: Most common isotope. It makes up about 99.3% of natural uranium.
- Uranium-235: Less common. It makes up about 0.7% of natural uranium.
- Uranium-234: Very rare. It is a decay product of Uranium-238.
Uranium-238 is not very radioactive. It decays very slowly. Uranium-235 is more important. It is used in nuclear reactors and bombs. It can sustain a chain reaction.
Isotopes differ in their stability. Uranium-238 has a half-life of 4.5 billion years. Uranium-235 has a half-life of 700 million years.
Understanding uranium’s atomic structure helps us grasp its density. Its dense nucleus and heavy isotopes make it a unique element. This is why uranium is so dense.
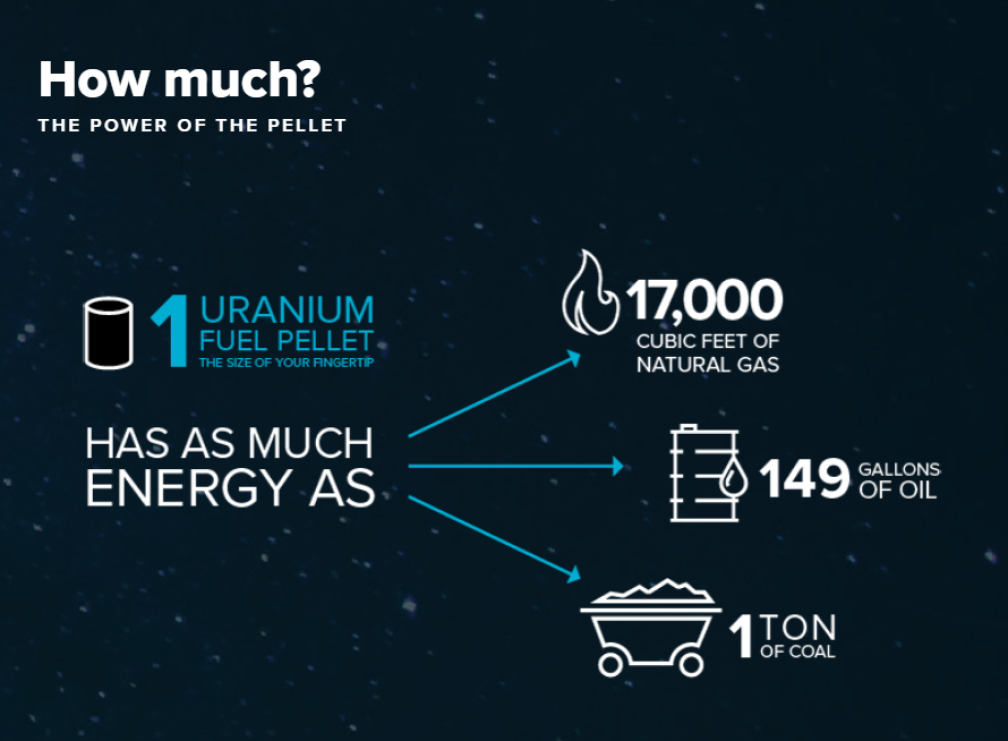
Credit: www.nek.si
Density And Atomic Weight
Understanding the density and atomic weight of uranium helps grasp its unique properties. These attributes make uranium vital in various fields, especially in nuclear energy.
Defining Density
Density measures how much mass fits into a certain volume. For uranium, this density is exceptionally high. At room temperature, uranium’s density is about 19.1 grams per cubic centimeter. This makes uranium about 1.6 times denser than lead.
In simpler terms, uranium is very heavy for its size. This high density is why uranium is used in applications needing heavy materials.
Measuring Atomic Weight
Atomic weight refers to the average mass of an element’s atoms. For uranium, the atomic weight is approximately 238.03 atomic mass units (amu). This is because uranium has different isotopes, mainly U-238 and U-235.
These isotopes have slightly different masses. U-238 is the most common isotope. It makes up about 99.3% of natural uranium. U-235, essential for nuclear reactors, is less abundant.
Let’s look at this information in a table:
| Isotope | Natural Abundance (%) | Atomic Mass (amu) |
|---|---|---|
| U-238 | 99.3 | 238.05 |
| U-235 | 0.7 | 235.04 |
Understanding these basic properties of uranium sheds light on its crucial role in science and industry.
Comparing Uranium
Uranium is a heavy metal. It is known for its high density. But how does it compare to other dense materials? Let’s look at how uranium stacks up against lead and gold.
Uranium Vs. Lead
Lead is another heavy metal. It is often used in batteries and shielding. Uranium, on the other hand, is denser than lead. Lead has a density of 11.34 g/cm³. Uranium’s density is 19.1 g/cm³. This makes uranium about 1.7 times denser than lead.
| Material | Density (g/cm³) |
|---|---|
| Lead | 11.34 |
| Uranium | 19.1 |
Uranium Vs. Gold
Gold is known for its value and density. It is often used in jewelry and electronics. Gold has a density of 19.32 g/cm³. Uranium is slightly less dense than gold. But their densities are very close.
| Material | Density (g/cm³) |
|---|---|
| Gold | 19.32 |
| Uranium | 19.1 |
Both lead and gold are dense materials. Yet, uranium’s density stands out. It is heavier than lead and almost as dense as gold. This makes uranium a unique and interesting element.
Applications Of Uranium
Uranium is known for its high density and unique properties. Its applications span various fields. The most significant uses are in nuclear energy and medical fields.
Nuclear Energy
Uranium plays a critical role in generating nuclear energy. Nuclear reactors use uranium as fuel. Uranium-235, an isotope, is especially important. It easily undergoes fission, releasing a large amount of energy.
Here’s a simple breakdown of uranium’s role in nuclear energy:
- Uranium-235 is enriched.
- Placed in a nuclear reactor.
- Undergoes fission, splitting atoms.
- Releases heat, producing steam.
- Steam turns turbines, generating electricity.
This process is efficient and produces a lot of energy. It is a key reason why nuclear power is a major energy source worldwide.
Medical Uses
Uranium is also valuable in the medical field. It is used in radiography and cancer treatment. Uranium’s radioactive properties help in diagnosing and treating illnesses.
Here’s how uranium is used in medicine:
- Medical imaging: Uranium aids in producing detailed images of internal organs.
- Cancer treatment: Radioisotopes of uranium target and destroy cancer cells.
- Sterilization: Medical tools are sterilized using uranium radiation.
These medical applications make uranium an essential element in healthcare.
Environmental Impact
Understanding the environmental impact of uranium is crucial. This dense metal affects ecosystems and human health. Let’s explore its effects in detail.
Mining Effects
Uranium mining disrupts the environment significantly. Large amounts of soil and rock are displaced. This process leads to habitat destruction. Water sources can get contaminated with toxic substances. Dust from mining sites can affect air quality. Local communities often face health risks.
Radioactive Waste
Radioactive waste is a major concern. Uranium mining and processing generate waste. This waste remains radioactive for thousands of years. Safe storage of radioactive waste is challenging. It requires secure and stable facilities. Groundwater contamination is a risk if storage fails. Long-term solutions for waste disposal are needed.

Credit: www.reddit.com
Frequently Asked Questions
What Is The Density Of Uranium?
Uranium has a density of 19. 1 grams per cubic centimeter.
How Does Uranium’s Density Compare To Gold?
Uranium is denser than gold. Gold has a density of 19. 3 grams per cubic centimeter.
Why Is Uranium So Dense?
Uranium atoms are heavy and tightly packed, leading to high density.
Is Uranium The Densest Element?
No, osmium and iridium are denser than uranium.
How Is Uranium’s Density Measured?
Density is calculated by dividing mass by volume.
Does Uranium’s Density Affect Its Usage?
Yes, its high density makes it useful in nuclear reactors and military applications.
Conclusion
Understanding uranium’s density helps us appreciate its uses in energy and industry. Its high density makes it valuable. Knowing these properties aids in safe handling and application. Whether for nuclear power or scientific research, uranium’s density is a key factor.
Stay informed and make wise decisions with this powerful element.
